
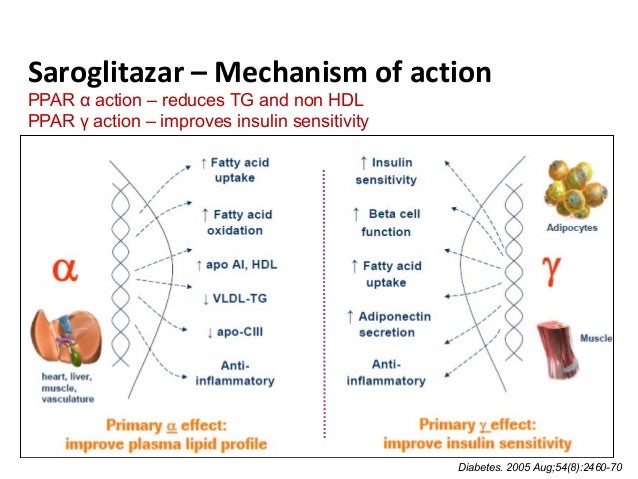
Idarucizumab binds dabigatran with a 350-fold higher affinity than thrombin ( Table 1).
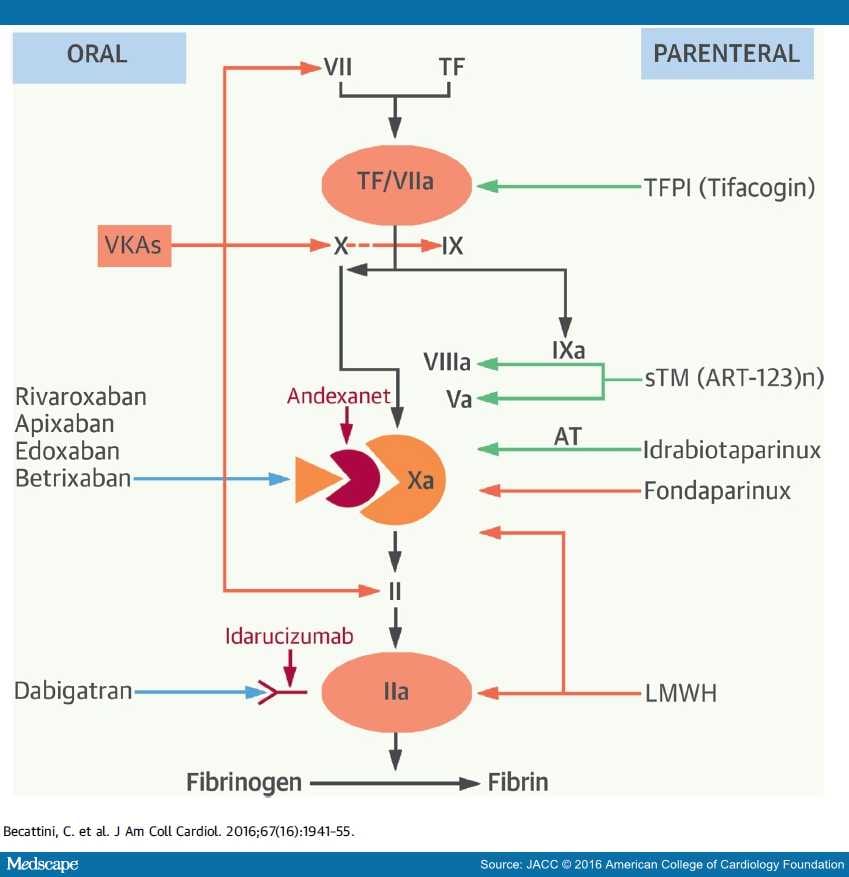
Hydrophobic interactions, H-bonds, and a salt bridge mediate the binding. The inhibition activity is due to the noncompetitive binding of dabigatran to a concave region present in the interface of the aDabiFab variable domain. In the original study, the parental mouse and humanized Fab antibodies showed similar in vitro inhibitory activity and clotting times, after the presence of dabigatran was reduced to baseline at antibody concentrations >10 nM, indicating complete neutralization of dabigatran at equimolar concentrations of dabigatran and aDabiFab. The variable region of the Fab has been humanized through a design and screening process. After that, the antibodies were humanized removing the variable regions from both heavy and light mouse antibodies’ chains and hybridized with human IgG constant regions. The original molecule has been produced from dabigatranhapten immunized mice. Citation6 Among them, idarucizumab is a specific reversal agent for dabigatran and is also the first one to receive approval from the US Food and Drugs Administration (FDA) and European Medicines Agency in 2015 ( Table 1). Citation5, Citation6 In order to face this issue, in the last years several companies started developing antidotes/reversal agents for NOACs. Citation4ĭespite several practical aspects that favor NOACs over warfarin (no need of continuous laboratory monitoring, limited drug–drug interactions, no food interactions, fixed-dose regimen, predictable anticoagulant activity, etc.), the absence of specific antidote and reversal agents has been a significant caveat to widespread use of these drugs. Dabigatran etexilate (aDabiFab) is the only direct thrombin inhibitor among the authorized NOACs and was the first one to be demonstrated more effective and safer than warfarin for AF Citation3 and has a similar efficacy and safety for VTE patients. In 2009 the introduction of non-vitamin K antagonist oral anticoagulants (NOACs) in the market as an effective and safer alternative for the prevention of thromboembolic events in atrial fibrillation (AF) patients Citation1 and the treatment and prevention of recurrences in patients with venous thromboembolic events (VTE) Citation2 drastically changed the current pharmacopeia and routine clinical treatment of these two conditions.


 0 kommentar(er)
0 kommentar(er)
